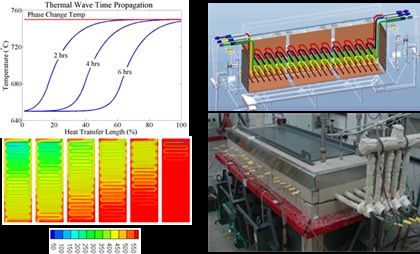
Thermal Hydraulics Laboratory
High Temperature Phase Change Thermal Energy Storage System Designs
Ionic liquids such as nitrates chlorides, carbonates and fluorides, have been shown to possess excellent heat transfer characteristics and can potentially be used as Phase change materials (PCM’s) at a variety of operating temperatures. The literature data on several of these salts can be found in several data bases and estimates of the thermal physical properties of mixtures of these salts can be determined based computational models (NIST), (Sohal, 2010) (Williams D.F., 2008),(Kaufman, 1970). Nitrate salts have been most widely considered with respect to solar applications due to their low melting points however they are typically limited to maximum operational temperatures around 600C, at or below the ARPA-E goals whereas, fluorides have typically been considered for nuclear applications due to their exceptional thermophysical properties (rcp product, k), high temperature stability and neutronics. Unfortunately, fluoride salts are typically the most corrosive and the most expensive. Therefore to achieve high exergenic efficiency, thermal storage capacity, cost, lifetime and commercialization potential it is necessary to also consider chlorides and carbonates.
Based on a thorough review with respect to cost (<$0.03/kJ), operating temperature (assuming outlet working fluid temperature constant at the melting point of the PCM) thermophysical properties and material corrosion (<0.1mm/year with 300 series stainless steel) three candidate salts have been chosen as the primary contenders for thermal energy storge operating at three different temperatures; Low temperature 640oC - KCL-CaCL2 (at% 25.9, 74.1), Mid temperature 723℃ - Li2CO3 and high temperature 801℃ - pure NaCl.